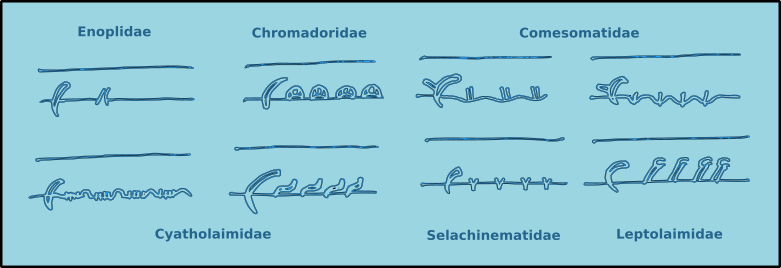
Introduction
Nematodes are probably the most abundant group within the meiofauna. Their total species diversity may well exceed that of the insects (Platt et al. 1984). They are ubiquitous in the benthic marine environment, found in the vast majority of micro-habitats, from the supralittoral down to the abyssal depths. There are currently 326 species recorded for Chile (Lee et al. 2008), the majority intertidal. Most of these species were described by Wolfgang Weiser (1953, 1954, 1956, 1959) working with material collected during the Lund Expedition to Chile (1948-49) (Brattström and Dahl 1951). More recent information comes from the work on the family Epsilonematidae by Lorenzen (1974) and Clasing (1980, 1983, 1986), the work of Chen and Vincx (1998, 1999, 2000a, 2000b) in the Magallanes region, and reports on the nematofauna of the Atacama Trench (Gambi et al. 2003)
Morphology
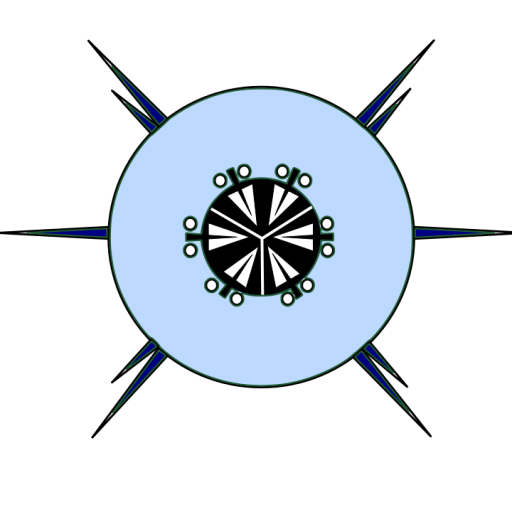
Many none specialists think that all nematodes look the same, but even a cursory look at the groups reveals that this is nonsense. Nematodes (Fig. 1) consist of a tubular body with an anterior and posterior end. The mouth is located terminally at the anterior end in most species. The exterior of the nematode is covered in a cuticle which may be smooth, but in the majority of free-living nematodes the cuticle is ornamented. Nematodes also have setae, sensory organs, on the surface of the body. In some species the setae are minute and found only at the anterior end, at the other extreme there are species completely covered in somatic setae, making them look hairy. Nematodes are dioecious, separate sexes, the males have a number of additional morphological characteristics, such as a spicule and precloacal supplements. Each of these morphological characteristics will be discussed in more detail below.
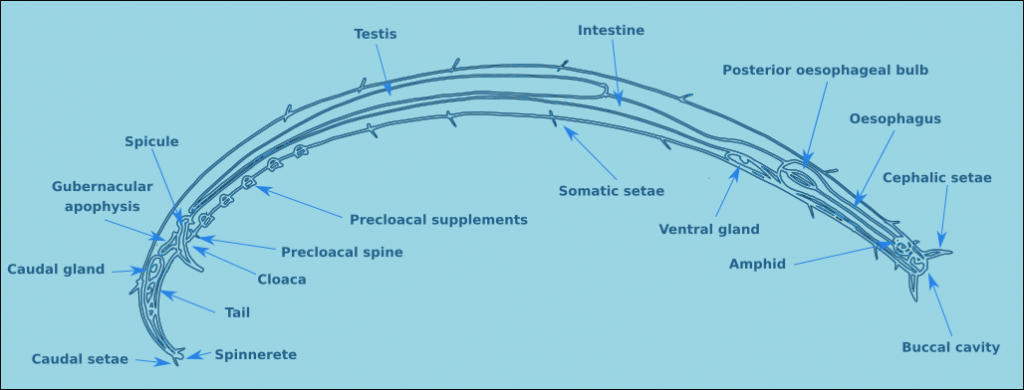
Figure 1. A generalised male nematode.
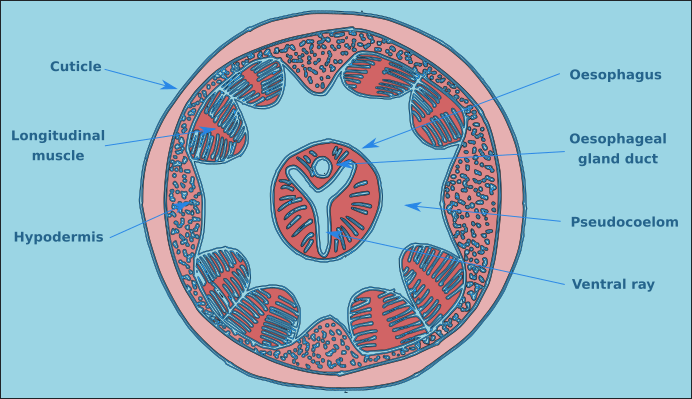
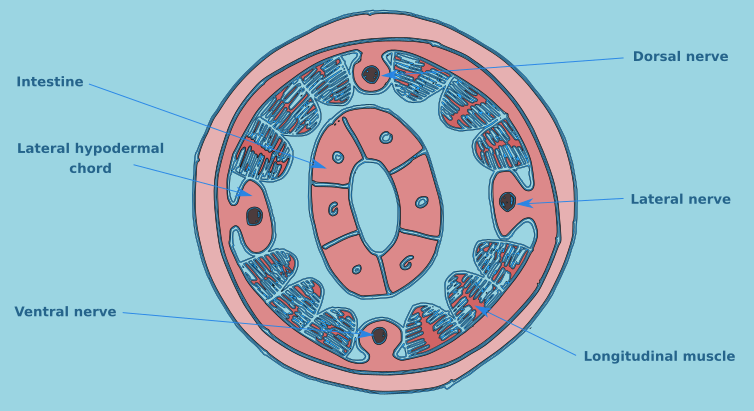
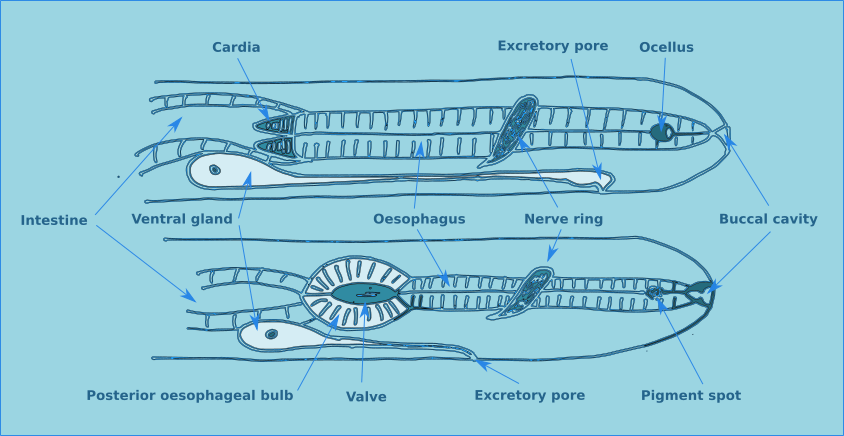
The structure of a nematode is essentially a tube within a tube. The external tube consists of, from the outside in, the cuticle, the hypodermis, and a layer of longitudinal muscles. Nematodes do not posses circular muscles, and as a result cannot lengthen or shorten their body length. Nematodes move by contracting alternate blocks of dorsal and then ventral longitudinal muscles. These muscle blocks act against a hydrostatic skeleton that results from the high turgid internal pressure in the pseudocoelom. These muscle contractions produce the characteristic serpentine form of locomotion observed in nematodes. These dorsal and ventral muscle blocks are also the reason why the majority of nematodes when fixed present in lateral view. The internal tube is the digestive tract. This starts terminally (usually) at the anterior end with the buccal cavity, this is followed by the oesophagus (Fig. 2) and the intestine (Fig. 3). The digestive tract ends subterminally with a short rectum and an anus or cloaca (males). As a result of the subterminal position of the anus, nematodes have a tail. Between the two tubes is the psuedocoelom, which is filled with a liquid. The pseudocoelom is “psuedo” because it lacks an epithelia lining. The reproductive organs (testes or ovaries) are found in this cavity.
Figure 2. Transverse oesophageal section.
Figure 3. Transverse intestinal section.
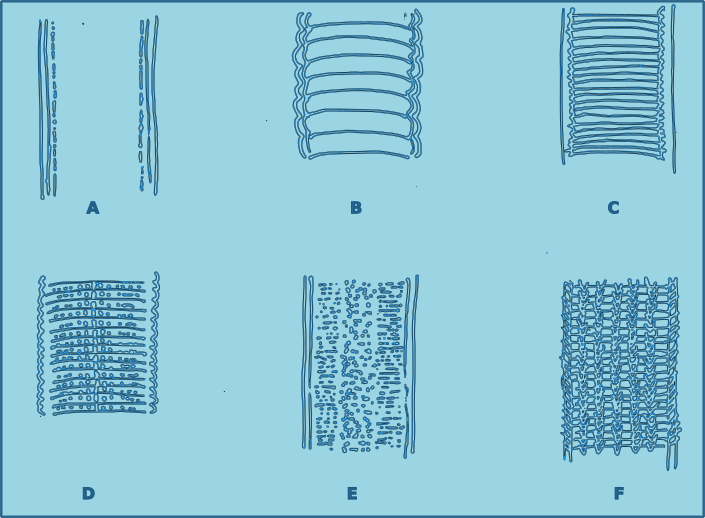
The cuticle of free-living nematodes shows a high degree variability between species, and is therefore a useful character for species identification. In some groups the cuticle is smooth, in others there is pseudo-annulation, in others the cuticle is highly ornamented, with dots, pores and or ridges. In some species this ornamentation is limited to lateral regions, in which case it is referred to as lateral differentiation. When the ornamentation is distributed uniformly it is referred to as homogeneous, and when the distribution changes along the length of the nematodes, the distribution is heterogeneous.
Figure 4. Cuticle morphology. A. Cuticle smooth without dots; B. Cuticle coarsely striated, resembling annulations; C. Cuticle externally fairly smooth but striations appear to be the result of deeper structure; D. Cuticle marked with transverse rows of dots; E. Cuticle covered with fine dots, sometimes irregular laterally, although the surface appears smooth; F. Cuticle marked with longitudinal rows of structures.
The cuticle of nematodes supports a series of different sensory structures known as sensilla (sensillum – singular). These sensilla all have the same basic structure, but differ in their morphology. The long sensilla are known as setae, and the shortest as papilla. Sensilla can be found all over the body of the nematode. Those found at the head end are known as cephalic setae. Those between the head and the mid part of the oesophagus are known as cervical setae. Those located on the tail are caudal setae, and on the tip of the tail, terminal setae. Setae located on the rest of the body are referred to as somatic setae, and they are sometimes organised in longitudinal rows.
Figure 5. Anterior view of the positions of the cephalic setae.
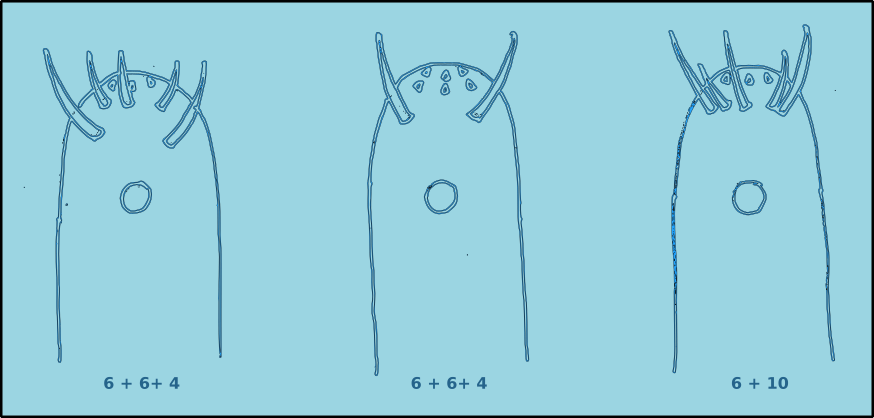
The positions of the sensilla on the head conforms to a specific pattern. There are six labial sensilla (two lateral and four submedian) positioned around the stoma (mouth). They are normally papilliform or very short setae. As a result they can be very difficult to observe in many species. Posterior to the labial setae are there is normally a ring of six external labial setae, and a circle of four cephalic sensilla, usually setae. The basic arrangement can be described as 6 + 6 + 4.
Figure 6. Lateral view of the positions of the cephalic setae.
In some species the external labial sensilla are papilliform, and as a result there appears to be only four cephalic setae. Another possible arrangement is where the four cephalic setae are positioned at the same level as the external labial sensilla, this arrangement is referred to as 6 + 10. In some species there maybe additional cephalic setae. The lengths of the cephalic setae are often cited as a proportion of the head diameter.
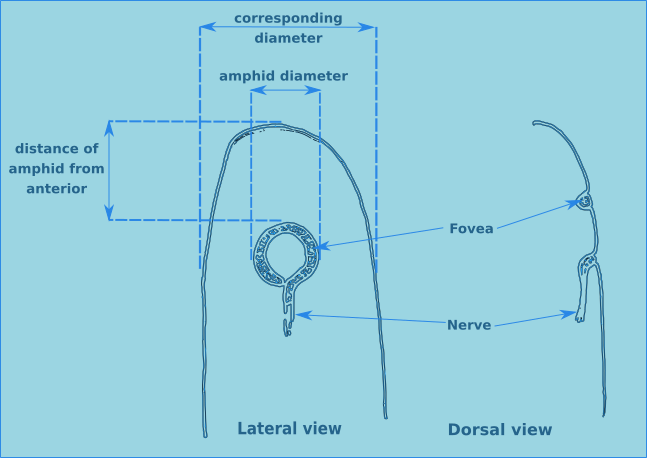
There are two other specialised sensilla located laterally on the head, the amphids (Figs. 7 & 8). These bilaterally symmetrical structures are of great importance in the identification of species. There are two basic types, spirals and nonspirals. Generally, a nonspiral amphid has a structure similar to a pocket or cup (Type A) where the external aperture takes the form of a transverse slit, which leads to a cavity (fovea) filled with a gelatinous substance.
Figure 7. Lateral and dorsal views of a generalised amphid.
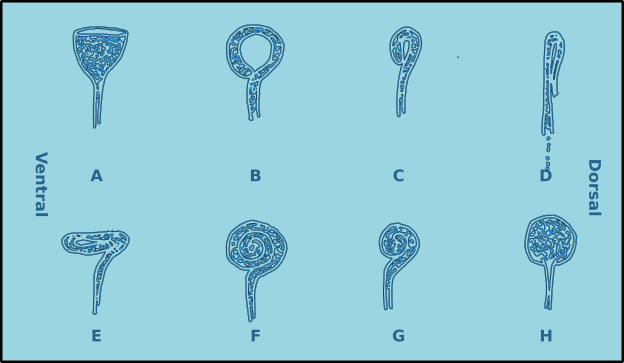
In a spiral amphid the fovea is open and elongated, and normally turns towards the ventral side (towards the nerve), though in a few species it may turn towards the dorsal side (Type D). When there is only one turn in the fovea than the amphid is of the loop type (Types B, C, D & E). If there are several turns then it is of the spiral type (Types F & G). Variations include transverse (Type E) and circular amphids (Type H) (Fig. 8). In the descriptions of species the width of the amphid is recorded as a proportion of the corresponding body diameter (c.b.d.) and the distance from the anterior of the head in terms of head diameters (h.d.) to the anterior margin of the amphid (Fig. 7).
Figure 8. Amphid morphological diversity. A. Pocket or cup shaped amphid (nonspiral); B. Loop shaped amphid (spiral); C. Small loop; D. Dorso-ventrally compressed loop (turns to the dorsal side); E. anterio-posteriorly compressed loop; F. Multispiral amphid; G. Multispiral amphid with fewer turns; H. Circular amphid.
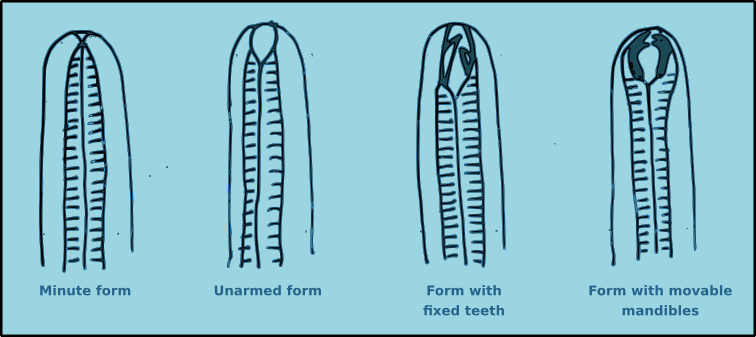
Nematodes exhibit a wide range of buccal morphologies (Fig. 9), which reflects the diversity of trophic guilds to which they belong. In many species the buccal cavity lacks hard structures an its size varies from minute to relatively large. In some species the buccal cavity contains immovable hard structures known as teeth. In other species there are movable hard structures known as mandibles. In yet other species there may be additional hard structures such as rows of small denticles. For many species the structure of the buccal cavity are important morphological characteristics for identification.
Figure 9. Generalised views of the principal buccal morphology types found in free-living nematodes.
Chromadorids often have complex buccal morphologies (Fig. 10). The mouth can contain rows of denticles, and or ruga, folds in the inner wall of the anterior portion of the buccal cavity, also referred to as the cheilostom. In some species, such as Hypodontolaimus, there may be a swelling of the oesophagus around the teeth, referred to as a buccal bulb.
Figure 10. A generalised buccal cavity of a Chromadorid.
The buccal cavity leads to the oesophagus which is the muscular anterior part of the intestine which functions to pump food to the main part of the intestine. The lumen of the oesophagus has a triradial transverse section (Fig. 2). The oesophagus can be cylindrical along its entire length, but in many species there is a muscular oesophageal bulb, in some species there are multiple bulbs.
Some nematodes, particularly those that live in phytal environments have paired pigment spots situated laterally on the cervical region. In some species these pigment spots have a basic lens, forming a ocella.
Figure 11. Miscellaneous morphological features found on the anterior end of free-living nematodes.
In general, approximately half way along the oesophagus there is a curcumesophageal commissure, more commonly known as the nerve ring. Its relative position is a useful characteristic for identifying some species.
An excretory system consisting of a ventral gland and a duct opening through a ventral pore is found in the oesophageal region. The ventral gland is conspicuous in some species but not others. The exact function of this gland is still not clearly understood.
In some species a muscular structure is found at the base of the oesophagus, the cardia. The intestine is a straight tube formed of a single layer of cells and has no taxonomic importance. The rectum connects the intestine with the anus in females, or the cloaca in males.
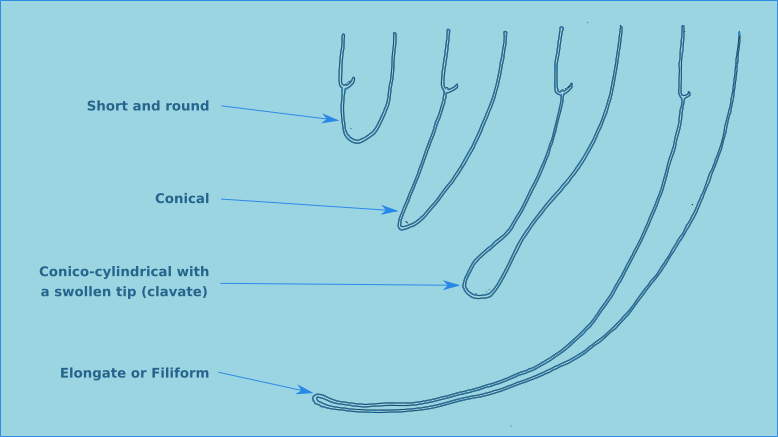
Figure 12. The four principal tail morphologies of free-living nematodes.
The tail morphology of free-living nematodes is highly variable, and therefore a usefull characteristic for species identification. There are four principal types: rounded, conical, conico-cylindrical, and elongate/filiform (Fig. 12). The length of the tail is recorded in terms of anal body diameter (a.b.d.). The tail contains unicellular caudal glands (Fig. 1), typically three. Which are normally confined to the tail, though in some species they can extend anteriorly of the anus. The caudal glands are responsible for secreting an adhesive which exits via a specialised structure at the tip of the tail called a spinneret (Fig. 1). Tail morphology can be used to assign nematodes to functional groups, for more information see Thistle and Sherman (1985).
Reproduction
Free-living nematodes have separate sexes (dioecious). The female has one or two ovaries (didelphic (A) or monodelphic (B)) which can be either reflexed or not (Fig. 12). The number and structure of the ovaries are useful characters for identification. The position of the vulva varies between species. In didelphic species the vulva is usually position somewhere around the mid-point of the body on the ventral side. In monodelphic species the vuva is positioned posteriorly close to the anus. The position of the vulva is expressed as a percentage of the body length from the anterior end (V%), except in species where the tail is extremely long (filiform) where the position of the vulva is expressed as a percentage of the body length to the anus (V’%).
Figure 12. Morphology of the ovaries of free-living nematodes.
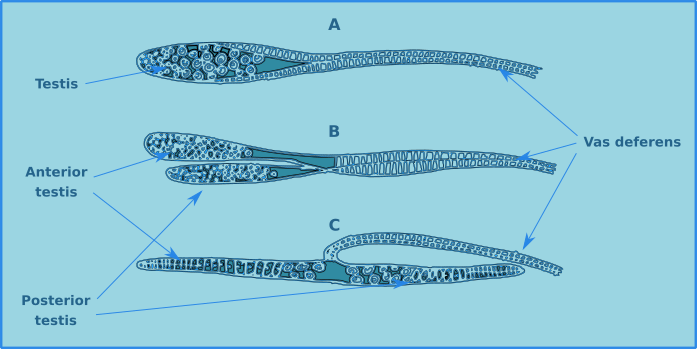
The males generally have two testicles (diorchic) which can be either opposed (C) or in tandem (B), however in some major groups there is only one testicle (Fig. 13). The testes can be difficult to distinguish in the body cavity, though in reality they are of little use in the identification of species.
Figure 13. Testes of free-living nematodes, single (A), diorchic in tandem (B), diorchic opposed (C).
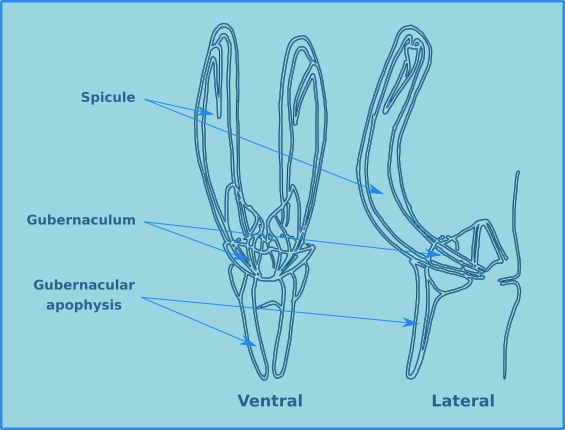
The male structures associated with copulation are extremely useful for the identification of specimens to species level. Male free-living nematodes have cuticularised copulatory structures and in many cases accessory structures. In general there are a pair of cuticuarised spicules and a guide piece called the gubernaculum (Fig. 14). These are found in a sac which opens on the dorsal side of the cloaca. The size and morphology of the spicules and the gubernaculum vary considerably between species. The gubernaculum in some species may also have an apophysis (an extension) which is usually projected posteriorly. Many species also have a number of pre-cloacal supplements (Fig. 15) which vary considerably in form and number. There may also be pre- or post-cloacal spines or setae, and in some species these may be joined by alae (wing like extensions) either side of the cloaca.
Figure 14. The morphology of the spicule and gubernaculum.
For free-living nematodes fertilisation is internal. The male holds the female using its tail and inserts the sperm into the vulva using the spicula. In the majority of species the female fixes the fertilised eggs in sticky capsules to grains of sand or other substrates. Some species display viviparity, where the larvae develop in the uterus. The nematodes develop through larval and juvenile stages that look very similar to the adults, but lack gonads and developed reproductive structures. Because of this it is often difficult to assign juveniles to a specific species.
Figure 15. Examples of the pre-cloacal supplements found in free-living nematodes.
Feeding
Habitat
Table 1. Abundance and diversity associated with different habitats.
Spatial Variation
Temporal Variation
Table 2. Life-cycle data for different species.
Taxonomy
Table 3. Nematode classification.
Chilean Species List
Current list of the free-living marine nematode species to be found in Chilean waters, ordered by Family.
References
Brattström, H. and Dahl, H. (1951) General account, lists of stations, hydrography. Reports of the Lund University Chile expedition 1948-49, 1. C.W.K. Gleerup, Lund, Sweden. 39 pp.
Chen, G. and Vincx, M. (1998) Nematodes from the Strait of Magellan and the Beagle Channel (Chile): description of four new species of the Comesomatidae. Hydrobiologia379: 97-118.
Chen, G. and Vincx, M. (1999) Nematodes from the Strait of Magellan and the Beagle Channel (Chile): the genus Sabatieria (Comesomatidae: Nematoda) with the description of Sabatieria coomansi n. sp. Hydrobiologia 405: 95-116.
Chen, G. and Vincx, M. (2000a) Nematodes from the Strait of Magellan and the Beagle Channel (Chile): the genera Cervonema and Laimella (Comesomatidae: Nematoda).Hydrobiologia 427: 27-49.
Chen, G. and Vincx, M. (2000b) New and little known Nematodes (Monhysteroida, Nematoda) from the Strait of Magellan and the Beagle Channel (Chile). Hydrobiologia429: 9-23.
Clasing, E. (1980) Postembryonic development in species of Desmodoridae, Epsilonematidae and Draconematidae. Zoologiche Anzeiger 204: 337-344.
Clasing, E. (1983) Leptepsilonema gen.n. (Nematoda, Epsilonematidae) from Chile and the Caribbean Sea. Zoologica Scripta 12: 13-17.
Clasing, E. (1986) Epsilonematidae (Nematoda) from Chiloe (southern Chile) with descriptions of two new species. Zoologica Scripta 15: 295-303.
Gambi, C., Vanrusel, A. and Danovaro, R. (2003) Biodiversity of nematode assemblages from deep-sea sediments of the Atacama Slope and Trench (South Pacific Ocean). Deep Sea Research I 50: 103-117.
Lee, M.R., Clarke, M., Fernandez, M.E., Gonzalez, C., Rozbaczylo, N., Valdovinos, C., Hermosilla, C., Prado, L. and Castilla, J.C. (2008) Diversity of free-living benthic marine invertebrates in Chile. Revista Chilena Historia Natural 81: 51-67.
Lorenzen, S., 1974. Glochinema nov. gen. (Nematodes, Epsilonematidae) aus Südchile.Mikrofauna des Meeresbodens 47: 393–410.
Platt, H.M., Shaw, K.M. and Lambshead, P.J.D. (1984) Nematode species abundance patterns and their use in the detection of environmental perturbations. Hydrobiologia118: 59-66.
Thistle D. and Sherman, K. M. (1985) The nematode fauna of a deep-sea site exposed to strong near-bottom currents. Deep-Sea Research. 32: 1077-1088.
Wieser, W. (1953) Free-living marine nematodes. I. Enoploidea. Reports of the Lund University Chile Expedition 1948-1949. 10. C.W.K. Gleerup, Lund. 155pp.
Wieser, W. (1954) Free-living marine nematodes. II. Chromadoroidea. Reports of the Lund University Chile Expedition 1948-1949. 17. C.W.K. Gleerup, Lund. 148pp.
Weiser, W. (1956) Free-living marine nematodes. III. Axonolaimoidea and Monhysteroidea. Reports of the Lund University Chile Expedition 1948-1949. 26. C.W.K. Gleerup, Lund. 115pp.
Weiser, W. (1959) Free-living marine nematodes. IV. General part. Reports of the Lund University Chile Wxpedition 1948-1949. 34. C.W.K. Gleerup, Lund. 111pp.