Presentation
Publication
Lee, M.R. & Mayorga-Dussarrat, J. (2016): The impact of feeding by Chilean flamingos (Phoenicopterus chilensis) on the meiofaunal assemblage of a tidal flat. Marine Biology Research, 12: 1039-105. DOI: 10.1080/17451000.2016.1228975
Theoretical Framework
Flamingos of the genera Phoenicopterus are large bodied birds that feed in estuaries and other protected coastal areas. As a result of their body size and high density populations thy can cause a significant impact on the benthic communities of these coastal habitats (Glassom & Branch 1997a, 1997b; Esté & Casler 2000). The effects of flamingos feeding in the benthic environment have been studied in South Africa (Glassom & Branch 1997a, 1997b), Venezuela (Esté & Casler 2000) and Chile (Cifuentes 2006). The Flamingos are bioperturbors of tidal flats and could potentially have a significant impact on benthic communities. The impact of Phoenicopterus chilensis (Molina 1782) (Fig. 1) on the macrofauna of Caulín Bay has been studied by Cifuentes (2006). This paper investigates the impact of feeding flamingos on meiofaunal assemblages.
Figure 1. Four Flamingos (Phoenicopterus chilensis, Molina 1782) feeding on the tidal flat at Caulín.
The Chilean Flamingo, P. chilensis, lives across a wide area of southern South America, including Chile, Argentina, Bolivia, Perú and Paraguay; and is an occasional visiter to Uruguay, Brazil, Ecuador and the Falkland Islands. A census undertaken in 2010 recorded 283,000 individuals, and the total population was estimated to be in the region of 300,000. The natural habitat of P. chilensis is tidal flats, estuaries, lagoons and salt lakes up to an altitude of 4,500 m. The conservation status of the Chilean Flamingo according to the IUCN is “near threatened, due to the collection of its eggs, ground water extraction, mining activity, and the general degradation and loss of its habitat (http://www.birdlife.org/datazone/speciesfactsheet.php?id=3770).
Caulín Bay (Fig. 1) is located on the north coast of the island of Chiloé, on the southern shore of the Chacao channel (41º 49′ 20” S, 073º 37′ 19” W). The bay measures 3,800 m by 1,600 m, and is almost closed due to the presence of an island and extensive sand banks, resulting in two narrow channels, 170 and 780 m respectively, connecting it with the Chacao channel. The extensive tidal flats within the bay are used by a wide variety of migratory birds as an area for feeding and roosting. The bay makes upa large part of the Caulín bird sanctuary. The Flamingos arrive in Caulín during the autumn and stay until the spring. The exact date of arrival is variable, during 2003 for example they arrived in April and stayed until December (Cifuentes 2006), during 2012 the Flamingos had left by October (personal observation MRL). During 2003 the maximum density of Flamingos in Caulín was 489 individuals, of which approximately three quarters were adults (Cifuentes 2006), during a sample visit in May of 2009 approximately 500 individuals were observed (personal observation MRL).
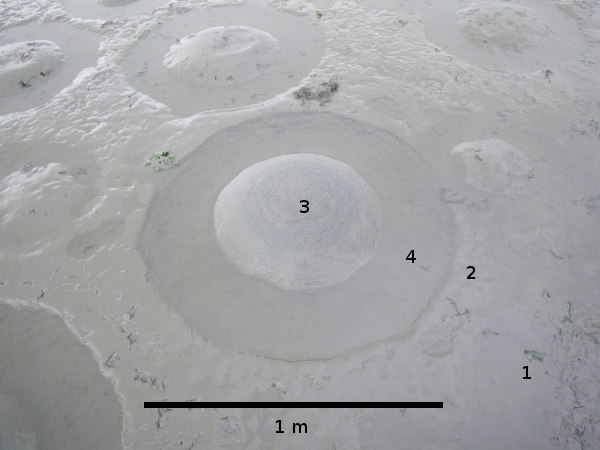
The Flamingos feed by filtering water and sediments with the lamellae of their beaks. The lamellae, in the case of P. chilensis, create gaps of between 80 and 900 µm, depending on their position, proximal or distal, in the beak (Mascitti & Kravetz 2002). In shallow waters (10 cm) the Flamingo walks in a circle suspending the sediment with up and down movements of the feet. At the same time the Flamingo submerges its head inverted in the water allowing it to filter the sediment suspension for prey items (http://www.youtube.com/watch?v=3XzsE75_TZw). The sediment once it has passed through the beak then falls away to one side. The result is the formation of a sediment feeding ring in the surface of the tidal flat, with a raised area of sediment in the center (Fig. 2). The sediment feeding rings can occur at densities of around 14 per 20 m2, and have a diameter of approximately 1 m (Cifuentes 2006).
[lang_enEvidence from the literature suggests that birds that feed on tidal flats in the main consume invertebrate prey with in the size range of macrofauna. However, it has been suggested that for some species meiofauna may form part of the diet. For example, Kuwae et al.(2008) reported that the Western Sandpiper (Calidris mauri) consumes the biofilm the grows on the surface of the sediment grains, and that this food source could provide up to 50% of its daily energy requirement. If birds are capable of consuming the biofilm, it is highly likely that they could also consume the meiofauna which are also feeding on the biofilm. Evidence also exists that small invertebrates can pass through the digestive tract of birds and survive. This has also been proposed as a method of dispersal for small invertebrates (Proctor & Moore 1965, Figuerola et al. 2005, van Leeuwen et al.2012). This evidence also suggests that small invertebrates, including meiofauna, are consumed by various species of birds. Determining if birds are specifically targeting meiofaunal prey of consuming them through non-targeted deposit feeding is difficult to determine. The only published research on the effects of Flamingos feeding activity on meiofauna was published by Glassom & Branch (1997b). They reported that their results provided ambiguous or nonexistent evidence of an impact by Flamingo feeding activity on meiofaunal assemblages. They did however note that the meiofaunal assemblage has a high capacity for rapid recuperation after a physical perturbation. Thus, it is possible that their experimental design did not have sufficient temporal resolution to detect the impact of the Flamingo feeding activity. When the Flamingos feed on the tidal flats and suspend the sediment there are two possible outcomes for the meiofaunal assemblage. The first is that the meiofauna are filtered out of the suspension and consumed by the Flamingo. The second is that the meiofauna are not filtered out of the suspension but are simply displaced to the boarders of the feeding ring by the feeding activity. In order to determine which of these hypothesis is the case it is necessary to take the field samples immediately after the perturbation event, as during the following flood tide the currents will begin to refill the feeding rings and repopulate the sediment with fauna.
Figure 2. Feeding ring produced by the Flamingos feeding at Caulín. It has an approximate diameter of 1 meter, with a mound of filtered sediment in the center. The numbers indicate the levels of bioturbation: 1 = undisturbed area; 2 = exterior border of the ring, lightly disturbed; 3 = interior border of the ring or mound, disturbed; and 4 = the feeding ring, highly disturbed.
As mentioned by Glassom & Branch (1997b) the meiofaunal assemblage has the capacity for rapid recuperation after a disturbance. This observation has been made frequently in the literature for a number of types of perturbation, physical (Johnson et al. 2007) to biological (Olafsson & Ndaro 1997), natural (Sherman et al. 1983) and anthropogenic (Fegley 1988). The majority of the meiofauna are not active swimmers and remain permanently in the sediment, the exceptions being small epibenthic crustaceans (Armonies 1988). Thus, it is assumed that the principal mode of dispersion for the meiofauna is in the majority of cases passive and dependent on random or stochastic events. Many meiofaunal species use strategies to maintain their position in the sediment, including the duo-gland system that permits individuals to adhere or detach from the sediment at will. Thus, when sediment is transported, by bottom currents for example, the meiofauna is transported along with it (Palmer 1988). Another possible mechanism, which functions at a smaller scale, is diffusion, that is the meiofauna move from one interstitial space to the next looking for new food sources. Both mechanisms provide a way for the meiofaunal assemblage to rapidly recolonise the perturbed sediment of the feeding rings.
Based on the description of the feeding mode of P. chilensis in Caulín, there are two possible hypotheses concerning the impact of Flamingo feeding activity on the meiofaunal assemblage. Hypothesis 1 – The Flamingos (P. chilensis) are feeding on the meiofaunal assemblage. Hypothesis 2 – The Flamingos (P. chilensis) are displacing the meiofaunal assemblage over short distances. If hypothesis 1 is correct we expect that the abundances of meiofauna in the feeding rings will be lower than the abundances in the boarders of the ring, which will in turn be lower than in adjacent unperturbed areas of the tidal flat. If hypothesis 2 is correct we expect that the abundances of meiofauna in the feeding rings will be lower than the abundances in the boarders of the ring, however the abundances in the boarders of the rings will be higher than the abundances in adjacent unperturbed areas of the tidal flat. The principal objective of this research was therefore to determine which of these two hypotheses is correct.
References:
Armonies, W. (1988) Active emergence of meiofauna from intertidal sediment. Marine Ecology Progress Series. 43:151-159.
Cifuentes, SL (2006) Efecto depredación/bioperturbación del Flamenco Chileno Phoenicopterus chilensis (Molina 1782) (Phoenicopteridae, Ciconiiformes) y la variabilidad espacio-temporal del macrobentos en una planicie intermareal de la Isla Grande de Chiloé, Sur de Chile. Tesis de Doctorado, Universidad Austral de Chile, Valdivia. 112 pp.
Esté, E. & Casler, C. (2000) Abundance of benthic macroinvertebrates in Caribbean flamingo feeding areas at Los Olivitos wildlife refuge, Western Venezuela. In: Waterbirds. Baldassarre, G., Arengo, F. & Bildstein, K. (eds). Special Publication 23:87-102.
Fegley, S. (1988) A comparison of meiofaunal settelment onto the sediment surface and recolonization of defaunated sandy sediment. Journal of Experimental Marine Biology and Ecology. 123:97-113.
Figuerola, J., Green, A. & Michot, T. (2005) Invertebrate eggs can fly: evidence of waterfowl-mediated gene flow in aquatic invertebrates. The American Naturalist. 165:274-280.
Glassom, D. & Branch, G. (1997a) Impact of predation by Greater Flamingos Phoenicopterus ruber on the macroinfuana of two southern African lagoons. Marine Ecology Progress Series. 149:1-12.
Glassom, D. & Branch, G. (1997b) Impact of predation by Greater Flamingos Phoenicopterus ruber on the meiofauna, microflora, and sediment properties of two southern African lagoons. Marine Ecology Progress Series. 150:1-10.
Johnson, G., Atrill, M., Sheehan, E. & Somerfield, P. (2007) Recovery of meiofauna communities following mudflat disturbance by trampling associated with crab-tiling. Marine Environmental Research. 64:409-416.
Kuwae, T., Beninger, P., Decottignies, P., Mathor, K., Lund, D. & Elner, R. (2008) Biofilm grazing in a higher vertebrate: the Western Sandpiper, Calidris mauri. Ecology. 89:599-606.
Mascitti, V. & Kravetz, FO. (2002) Bill morphology of South American Flamingos. The Condor. 104:73-83.
Olafsson, E. & Ndaro, S. (1997) Impact of the mangrove crabs Uca annulipes and Dotilla fenestrata on meiobenthos. Marine Ecology Progress Series. 158:225-231.
Palmer, M. (1988) Dispersal of marine meiofauna: a review and conceptual model explaining passive transport and active emergence with implications for recruitment. Marine Ecology Progress Series. 48:81-91.
Proctor, V. & Malone, C (1965) Further evidence of the passive dispersal of small aquatic organisms via the intestinal tract of birds. Ecology. 46:728-729.
Sherman, K., Reidenaur, J., Thistle, D. & Meeter, D. (1983) Role of a natural disturbance in an assemblage of marine free-living nematodes. Marine Ecology Progress Series. 11:23-30.
van Leeuwen, C., van der Velde, G., van Groenendael, J. & Klaassen, M. (2012) Gut travelers: internal dispersal of aquatic organisms by waterfowl. Journal of Biogeography. 39:2031-2040.