Introduction
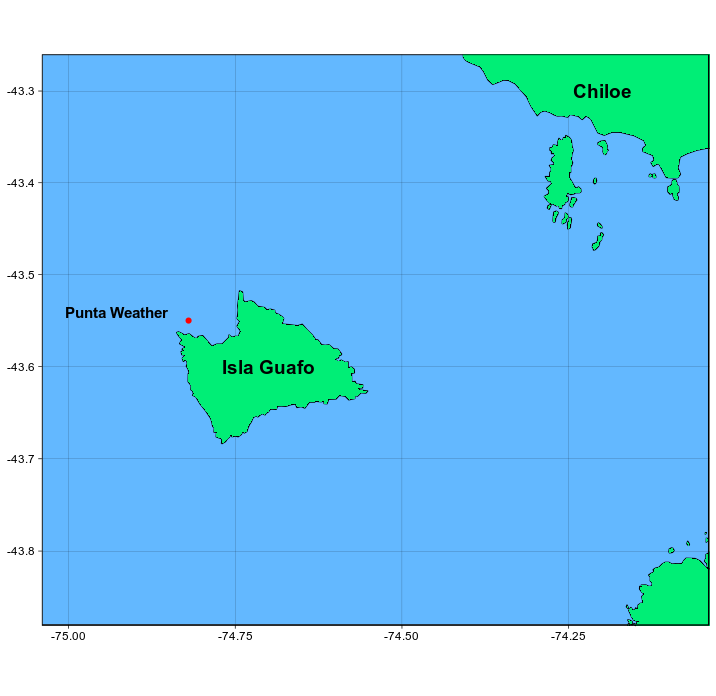
The Mystacocarida are a small distinct group of interstitial crustaceans of Subclass Maxillapoda, which includes the harpacticoid copepods, the only other group of interstitial crustaceans with which mystacocarids could be confused. The first mystacocarid species Derocheilocaris typicus was described by Pennak & Zinn in 1943 from the interstitial fauna of sand beaches on the northeastern coast of the United States. World wide 12 species have been described, divided between two genera, Derocheilocaris and Ctenocheilocaris (Hessler 1988). In Chile, Derocheilocaris galvarini was described by Dahl (1952) using material collected during the Lund University expedition to Chile (Brattström & Dahl, 1951). The type location was coarse sand from a depth of 25 m at an anchorage near Punta Weather on the north west side of Isla Guafo (43.55 °S, 74.82 °W) (Figure 1). The species was named in honour of the Chilean Naval ship Galvarino. The only other paper that discusses Chilean Mystacocarida is by Noodt (1961) in which he describes D. galvarini from medium intertidal sands in Las Cruces, on the Litoral Central coast. In 1976 Renaud-Mornant assigned D. galvarini to the genus Ctenocheilocaris. C. galvarini is found primarily in southern Chile, south of 32°, in the intertidal of intermediate beaches, most likely distributed between the level of the water-table and the surface, but avoiding dry sand.
Figure 1. Type location for C. galvarini near Punta Weather on the north west side of Isla Guafo.
Morphology
Mystacocarids are small crustaceans, typically less than 0.5 mm in length. They have a flexible vermiform body with 11 free body segments. The anterior end has long antennae and a primitively branched mandible. The posterior end is equipped with conspicuous claw-like caudal rami (Giere, 2009).
Figure 2. Photograph of a specimen of Ctenocheilocaris galvarini from Ritoque.
Lombardi and Ruppert (1982) made a detailed study of the movement of mystacocarids. Locomotion in Mystacocarida is fully dependent on the interstitial environment. With the long exopodites of the antenna and mandible pushing dorsally and the endopodite of the same appendages pushing ventrally, the organism moves through the interstitial spaces.
Reproduction
The mystacocarid reproductive cycle has not been well documented. However, the cycle is conservative, i.e. mystacocarids are direct benthic recruiters. Ontogenesis is abbreviated with the omision of the naupliar stage.
Feeding
Assumed to be grazers of the biofilm on the surface of sand grains.
Habitat
Samples collected recently (during the sampling for Fondecyt project 108033) allow the habitat of C. galvarini to be described with more certainty. The original type habitat was coarse sand at a depth of 25 m. The current sampling regime was confined to intertidal habitats so for the moment it is unknown whether C. galvarini is common in the sub-tidal or only occasional, Dahl (1952) refers to ‘numerous specimens’ but does not give an abundance figure. However, in none of the samples from the recent sampling trips was C. galvarini found associated with coarse sediment. Noodt (1961) describes C. gavarini from intertidal medium grained sediments, from a sediment depth of 20 cm and an abundance of one adult and two juveniles, he does not state the sample volume. Noodt’s (1961) sample was from Las Cruces, though he does not specify which beach or give precise coordinates, however, based on the site description it is likely that he is referring to the large intermediate beach Playa Grande which extends from the south side of Las Cruces down to Cartagena, where C. galvarini has been observed more recently. The abundance data presented here are all from quantitative samples collected in the retention zone between 0 and 10 cm depth. C. galvarini was also recorded in qualitative samples from the zones of retention (Tirua), resurgence (Tirua, Chaihuin) and sub-littoral fringe (Tirua), as well as from sediment in a temporary back-shore lagoon (Tirua) and in samples from the depth of the water-table (Calfuco and Cucao, depth approximately 30 cm at both sites). All samples, both quantitative and qualitative, that contained C. galvarini came from beaches with intermediate morphology (Short and Wright 1983) and medium sand (Wentworth scale, equates to a mean sediment grain size of 500 µm or +1 on the φ scale). Based on these observations the typical intertidal habitat of C. galvarini can be described as intermediate beaches, the majority of which have medium sand. The depth in the sediment is probably below the surface down to the interface with the water-table. It is likely that the low abundances observed in the majority of the quantitative samples was due to C. galvarini being present deeper in the sediment than the sampling depth of the corer. It is notable that the highest abundances were found a Llico where the water table was shallow, at around 20 cm deep, making it more likely that C. galvarini would be sampled by the quantitative methodology. Noodt’s (1961) conclusion was that C. galvarini was distributed sub-tidally and only occasionally found in the intertidal. The currently available information, however, suggests the reverse, that C. galvarini is an intertidal species that is found occasionally in the sub-tidal, though clearly more sub-tidal sampling is required.
Spatial Variation
C. galvarini was found at only nine of the 66 sites sampled, during Fondecyt project 1080033, along the exposed coast of Chile between 18 °S and 42 °S. Specimens were found in; quantitative samples from Matanzas, Bucalemu, Iloca, Llico, Calfuco, and Chaihuin; and qualitative samples from Tirua, Calfuco, Chaihuin, Tril Tril, and Cucao (Figure 3). C. galvarini have also been observed in samples from Las Cruces and Ritoque, but not during the sampling mentioned above.
Figure 3. The current known locations of C. galvarini along the Chilean coast.
Mystacocarida are, like the majority of the meiofauna, direct benthic recruiters, lacking a pelagic larval phase. Given the patchy distribution of C. gavarini an interesting question for future research is how do they disperse. The assumption is that transport of individuals between beaches is either the result of random events such as rafting or long-term dispersion through interconnected, sub-tidal patches of suitable habitat. Given also, the extreme morphological conservatism (Hessler, 1988) of the Mystacocarida these questions should ideally be examined with the aid of molecular techniques.
Temporal Variation
There are currently no studies investigating the temporal variation of Mystacocarida in Chilean beaches.
Taxonomy
Phylum: Arthropoda
SubPhylum: Crustacea
Class: Maxillopoda
SubClass: Mystacocarida
Order: Mystacocaridida
Family: Derocheilocarididae
Genera: Ctenocheilocaris; Derocheilocaris
Chilean Species List
Only one recorded species:
Ctenocheilocaris galvarini, Dahl 1952
References
Brattström, H. and Dahl E. (1951) General account, lists of stations, hydrography. Reports of the Lund University Chile Expedition 1948-1949. 1. C.W.K. Gleerup, Lund, Sweden.
Dahl, E. (1952) Mystacocarida. Reports of the Lund University Chile Expedition 1948-49.7. C.W.K. Gleerup, Lund, Sweden.
Giere, O. (2009) Meiobenthology. Springer-Verlag, Berlin. 527pp.
Hessler, R.R. (1988) Mystacocarida. In: Higgins RP and Thiel H (eds). 134-160.Introduction to the study of meiofauna. Smithsonian Institution Press, Washington DC.
Lombardi, J. and Ruppert, E.E. (1982) Functional morphology of locomotion inDerocheilocaris typica (Crustacea, Mystacocarida). Zoomorphology 100:1-10.
Noodt, W. (1961) Estudios sobre crustaceos chilenos en aguas subterreneas. IV. Nuevo hallazgo de Derocheilocaris galvarini Dahl en Chile Central (Crustacea, Mystacocarida).Investigaciones de Zoologia Chileana 7: 97-99.
Pennak, R.W. and Zinn, D.J. (1943) Mystacocarida, a new order of Crustacea from intertidal beaches in Massachussetts and Connecticut. Smithsonian Miscelaneous Collections 103: 1-11.